KATP channels are present in many areas of the body and play a role in managing many normal functions, including hunger/satiety and mood, insulin secretion regulation and limiting the accumulation of excess fat.
Our lead product candidate, DCCR (Diazoxide Choline) Extended-Release tablets, is a proprietary extended-release formulation of a crystalline salt of diazoxide. DCCR has demonstrated the ability to activate the KATP channel in the brain, pancreas and fat tissue, which may:
- Help regulate appetite and reduce hyperphagia
- Improve other difficult to manage behaviors
- Reduce resistance to insulin and, potentially, leptin, hormones that help regulate appetite
- Help limit the accumulation of excess body fat.
About DCCR
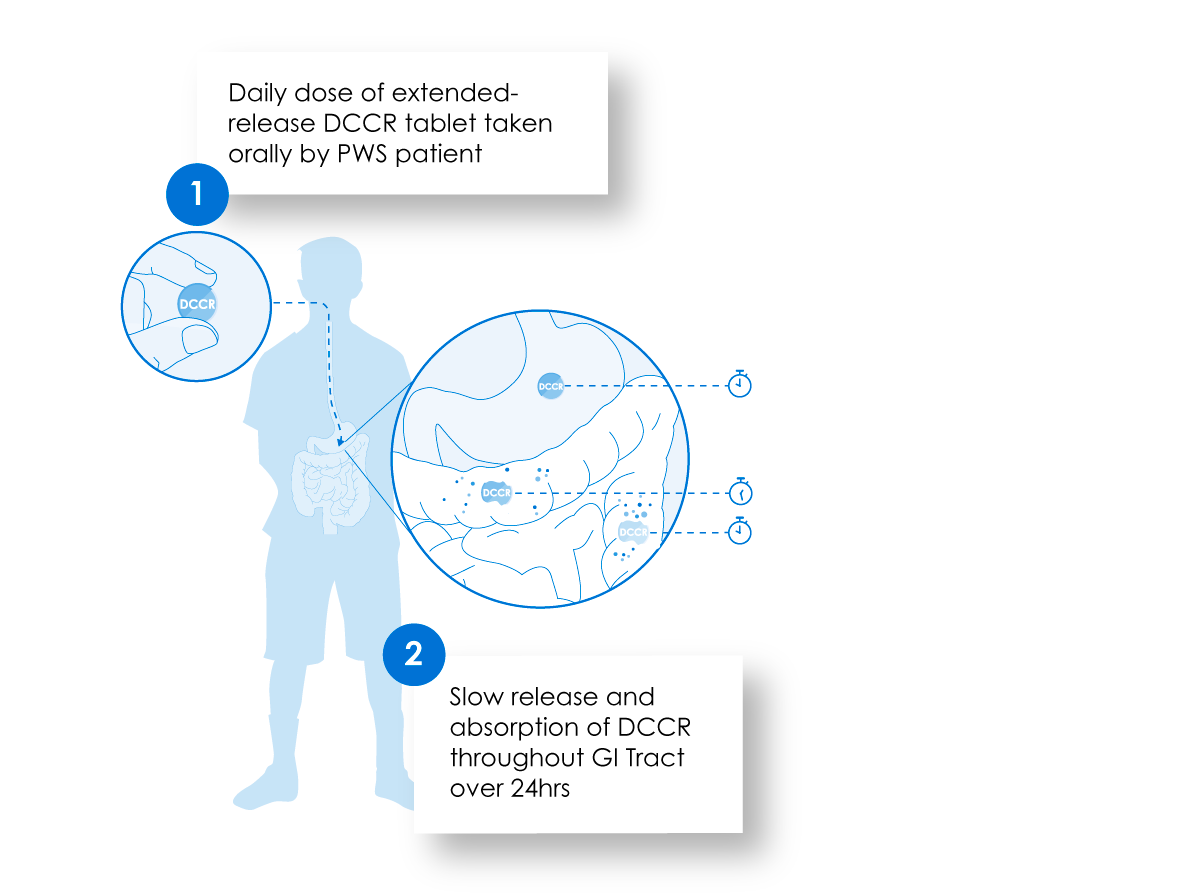
Our proprietary, extended-release formulation of DCCR allows the active drug to be released and absorbed for up to 24 hours beginning in the stomach and continuing in the small and large intestines. This helps maintain stable circulating levels of the drug with once daily dosing.
Mode of Action of DCCR in Prader-Willi Syndrome
DCCR, an investigational product, contains a crystalline salt of diazoxide formulated in a proprietary, extended-release formulation. This formulation allows the active drug to be released and then absorbed for up to 24 hours beginning in the stomach and then continuing in the small and large intestines. This helps maintain stable circulating levels of the drug with once daily dosing.
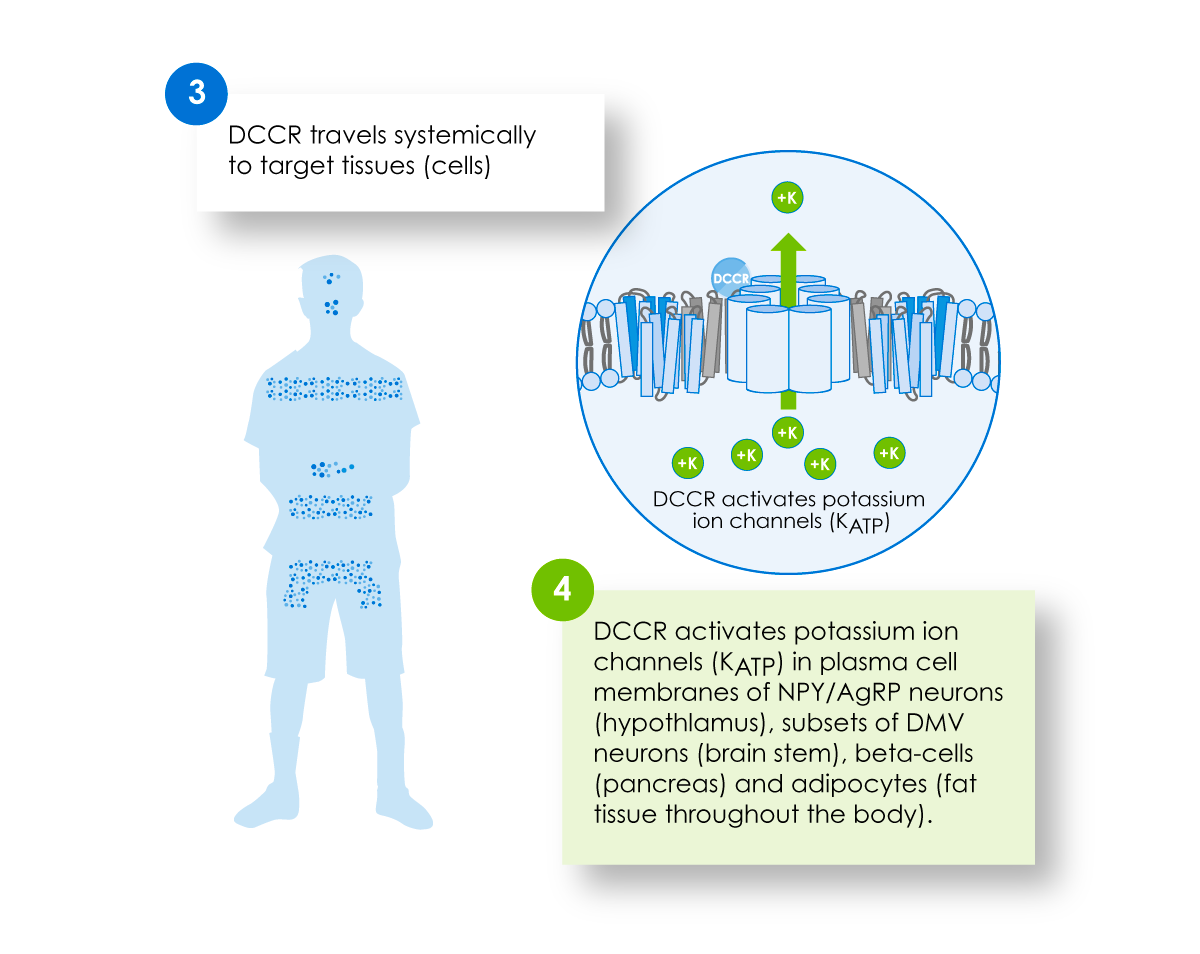
Active drug from DCCR has the potential to activate the KATP channel in the hypothalamus, other sites in the central nervous system, the pancreas and fat tissue. This may amplify the ability of leptin and insulin to regulate appetite, may modify behavior and may reduce body fat.
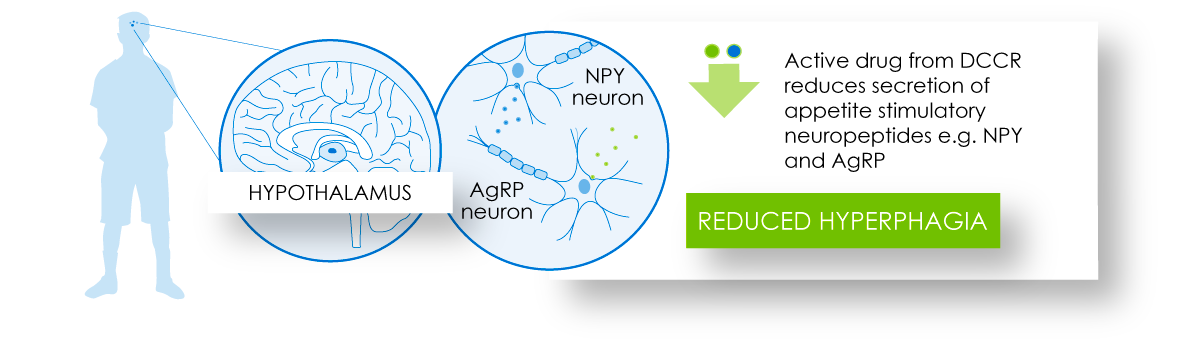
Active drug from DCCR is believed to activate KATP channels in the NPY/AGRP neurons in the hypothalamus, thereby reducing secretion of appetite stimulatory neuropeptides and neurotransmitters.
Administration of DCCR leads to significant reductions in circulating leptin levels. These decreases may represent a lowering of leptin resistance which may also help to reduce hyperphagia.
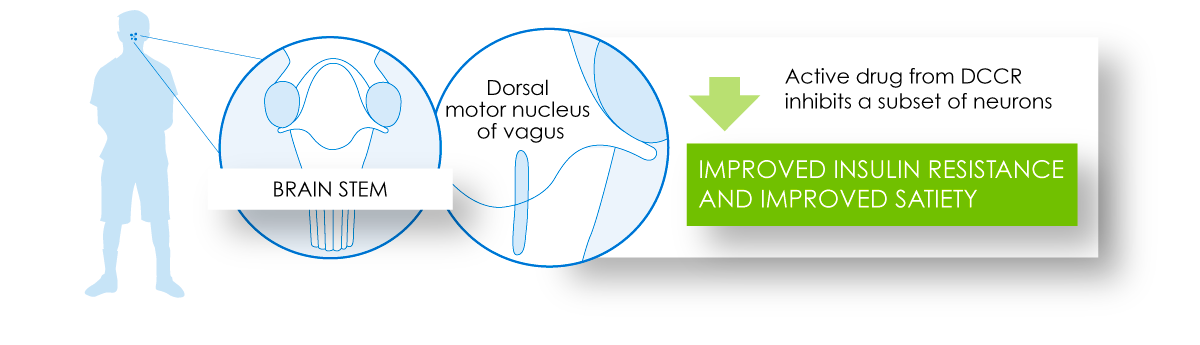
The central appetite regulatory effects of DCCR treatment are complemented by the potential action of the active drug in the dorsal motor nucleus of the vagus (part of the brain stem), which can contribute to improved satiety and insulin sensitivity.
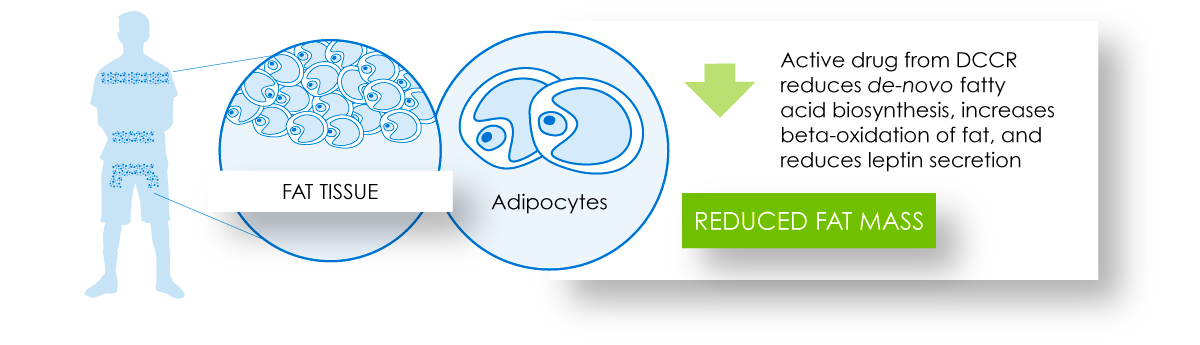
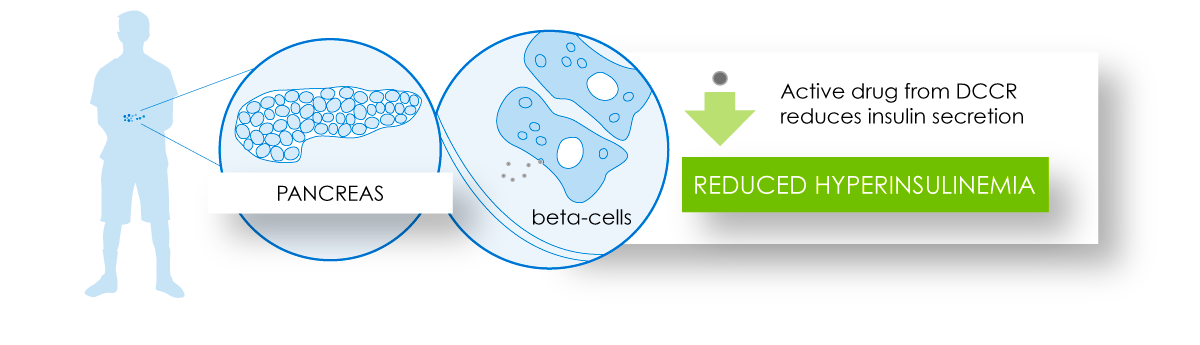
DCCR administration through its action in the pancreas and fat tissue has the potential to contribute to reduced fat accumulation and improved insulin resistance.
Clinical research with DCCR in people with Prader-Willi syndrome has shown sustained improvements in hyperphagia and other behavioral complications of this syndrome. Improvements in several metabolic and hormonal parameters were also noted.
Targeting other rare genetic forms of hyperphagic obesity
Other rare genetic forms of obesity appear to result from disruption of the regulation of appetite stimulatory neurons. These include SH2B1 deficiency obesity, obesity associated with PCSK1 mutation (rs6232 variant), and SIM1 deficiency obesity. In some forms of rare genetic obesity, there may be very marked dysregulation of the appetite suppressive signalling pathway, as occurs in MC4R deficient obesity. DCCR may have the potential to be an effective treatment for these conditions.
A clinical study of DCCR in SH2B1 deficiency obesity, SIM1 deficiency obesity and obesity associated with the rs6232 variant of PCSK1 is in the planning stage.